(1) Taha Laroussi, Laboratoire d’Hydrodynamique (LadHyX), CNRS, Ecole Polytechnique, Institut Polytechnique de Paris, 91120 Palaiseau, France;
(2) Mojtaba Jarrahi, Universit´e Paris-Saclay, CNRS, FAST, 91405 Orsay, France;
(3) Gabriel Amselem, Laboratoire d’Hydrodynamique (LadHyX), CNRS, Ecole Polytechnique, Institut Polytechnique de Paris, 91120 Palaiseau, France.
Table of Links
Discussion, Acknowledgements and References
III. RESULTS
A. First experimental results
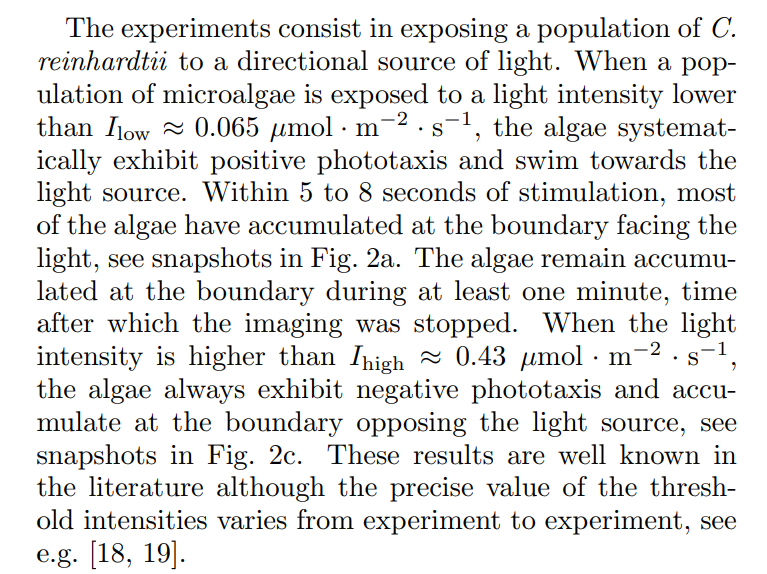
In between these two light intensities, there is a transition regime, where the phototactic behavior is hard to reproduce, despite keeping all experimental parameters identical: the microalgae sometimes exhibit a transient positive phototaxis followed by a negative phototaxis (see Fig. 2b), but can also display purely positive phototaxis, or purely negative phototaxis (see later in text). The aim of our work is to better understand the parameters responsible for this variety of algal behaviors in the transition regime. We investigate the effects of well diameter, algae concentration, light intensity, and finally history of the algae, on the phototactic behavior.
B. Quantification of the phototactic behavior
To quantify the phototactic behavior of a microalgae population constrained in its well, we binarize the experimental images using a simple Otsu threshold on pixel intensities. This leads to images where the algae are white, on a black background (see Supp. Fig. 1). In each image, we then calculate the center of mass zcm of the white pixels, corresponding to the center of mass of the population. The position of the center of mass is tracked over time, and eventually normalized to the radius of the well. A value of the center of mass zcm = 1 (resp. zcm = −1) corresponds to all algae accumulating at the boundary of the well facing (resp. opposite from) the light.
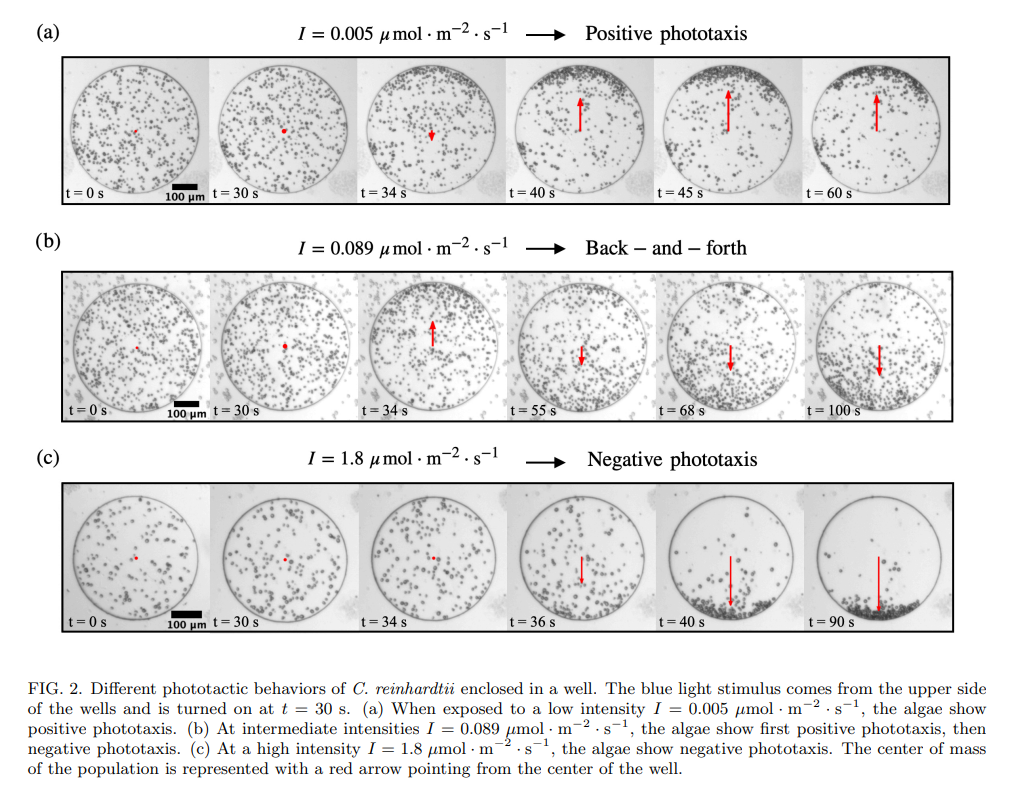
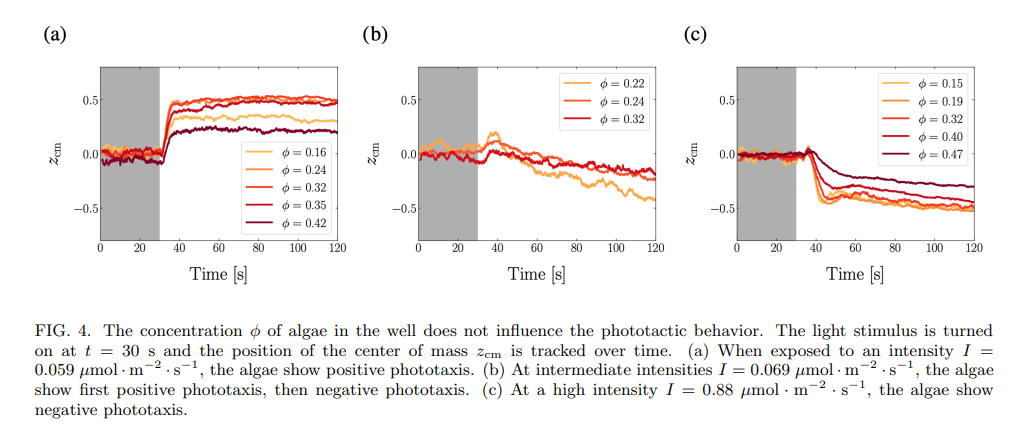
In one experimental run, the phototactic response of 15 – 30 wells containing microalgae is quantified. The field of view contains wells with at least 3 different diameters, and there are at least 4 wells of each diameter, see Fig. 1b. We start with an experiment where the algae are at the same concentration in all wells, and do not find any impact of the well diameter on the phototactic behavior of populations of C. reinhardtii: for all diameters, in one given experiment, the algae exhibit the same behavior, as shown by the evolution of the center of mass of the population, see Fig. 3. Note that the center of mass never reaches ±1. This is mainly due to a fraction of the algae not responding to light, see Supp. Fig. 2. A second-order effect is that algae take space, and so the center of mass of the population can never reach ±1.
The time scales for pure positive and pure negative phototaxis are different: positive phototaxis leads to an accumulation on the side of the light source within tpos ≈ 5.3 ± 0.9 s (mean ± std. deviation) after the stimulus is turned on. The time scale for negative phototaxis is slightly longer: tneg ≈ 9.1 ± 1.1 s. In the case where there is a back-and-forth between positive and negative phototaxis, the first accumulation occurs within tpos ≈ 5.1±0.3 s while the second accumulation is within tneg ≈ 34 ± 10 s. Negative phototaxis always occurs on a time scale longer than positive phototaxis, irrespective of the behavior (back-and-forth or not) of the population.
C. Effect of cell concentration
One possibility for the alternating phototactic behavior would be to invoke screening of the light by the algae. We would then expect that at high algae concentration, the algae closest to the light source screen the light intensity, so that the algae further away from the light source see a dimmer light. This could result in a seemingly biphasic behavior, with, at high light intensities, algae close to the light source showing negative phototaxis, and algae further away from the light source exhibiting positive phototaxis.
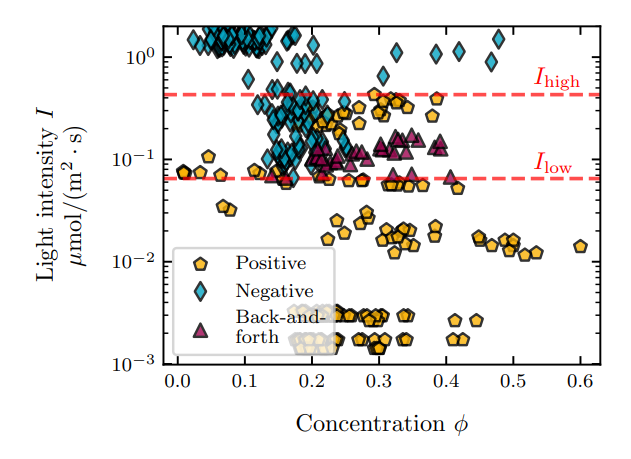
To test this hypothesis, we enclosed microalgae at concentrations ϕ = 0.008 to ϕ = 0.6 in the microwells, and monitored the evolution of their center of mass. The concentration ϕ is defined as the surface fraction of algae in a microwell, see Methods. For ϕ > 0.5, the well is essentially packed with algae, which impedes their motion and so leads to the center of mass not moving even during the light stimulus, see Supp. Fig. 3. For ϕ ≤ 0.5, we do not see any effect of the concentration on the sign of phototaxis: the phototactic behavior depends solely on the light intensity, see Fig. 4. There is however an effect of concentration on the position of the center of mass zcm: when the concentration is higher, the algae take more space and the center of mass of the population gets closer to the center of the well, see Fig. 4a and c.
The experiments were reproduced over a range of light intensities and algae concentrations, in more than 300 wells. In each well, the behavior was quantified as positive phototaxis, negative phototaxis, or back-and-forth. The resulting phase diagram of phototactic behavior as a function of light intensity and algae concentration shows no clear influence of the algae concentration on the phototactic behavior, see Fig. 5.
D. Effect of history
We finally investigated the effect of history on the behavior of the algae. To do so, a population of algae was kept in the dark for one hour and then exposed to a given light stimulus during 90 seconds. The light was then turned off during 90 seconds, after which the exact same stimulus was re-applied. At low light intensities, the qualitative behavior of algae does not change between the two stimuli: the algae always exhibit positive phototaxis, see the first two graphs in Fig. 6a. At high light intensities, the algae always exhibit negative phototaxis, see the first two graphs in Fig. 6c. At intermediate light intensities however, the behavior of the algae changes between the two stimuli: in the first stimulus, the algae exhibit a back-and-forth motion, first towards the light and then away from it. During the second stimulus, the algae show negative phototaxis, see the first two graphs in Fig. 6b.
To understand whether the change in behavior was permanent, the algae were allowed to rest in the dark for 30 minutes after the end of the second stimulus. Then, the same protocol was followed: a third stimulus, of the same intensity as the two previous ones, was applied. After 90 seconds in the dark, the algae were restimulated with a fourth, identical stimulus. In the case of low and high light intensity, the qualitative response of the algae was the same for the third and fourth stimuli as for the two first ones, see the last two graphs in Fig. 6a,c. At intermediate light intensities, the algae showed positive phototaxis during the third stimulus, and then qualitatively changed their behavior during the fourth stimulus to exhibit slightly negative phototaxis, see the last two graphs in Fig. 6b. This last response to light, averaged over 31 wells, is quite subtle. Indeed, after several stimuli, most of the algae stick to the wells, a likely effect of light stimulation [20]. These stuck algae move very slowly by gliding [21], see Supp. Fig. 4. The center of mass of the population then largely reflects the position of the stuck algae. The motile algae however do exhibit negative phototaxis, see Supp. Fig. 4.
Note that the quantitative response of the algae, as measured by the position of the center of mass zcm, differs between the four stimuli – even in the case of low and high light intensities. For example, at low light intensities (positive phototaxis), the shift in the center of mass is identical for stimuli 1 and 2. The shift is less pronounced for stimuli 3 and 4, see Fig. 6a. In contrast, in the case of negative phototaxis, the shift of the center of mass is identical for stimuli 1 and 3, as well as for stimuli 2 and 4, see Fig. 6c: there, resting in the dark for 30 minutes seems to reset the phototactic behavior.
What is important in our case however is the qualitative response: the algae can change phototactic behavior at intermediate light intensities due to the stimuli they previously experienced. The change in behavior is not an adaptive response, which would make the algae show first negative phototaxis, then positive phototaxis in response to the same stimulus. It rather corresponds to an integration of the signal over time. This effect explains the variety of behaviors observed in the transition regime between positive and negative phototaxis: one cannot predict the phototactic response of an alga without knowing its history.
This paper is available on arxiv under CC 4.0 license.